Featured
Atrial Septal Occluder
Postprocedure device thrombosis is rare. Assess clinical performance of a new device for transcatheter closure of atrial septal defect ASD.
 Amplatzer Septal Occluders For Asd Closure Abbott
Amplatzer Septal Occluders For Asd Closure Abbott
The AMPLATZER Septal Occluder is a percutaneous transcatheter atrial septal defect closure device intended for the occlusion of atrial septal defects ASD in secundum position or patients who have undergone a fenestrated Fontan procedure and who now require closure of the fenestration.
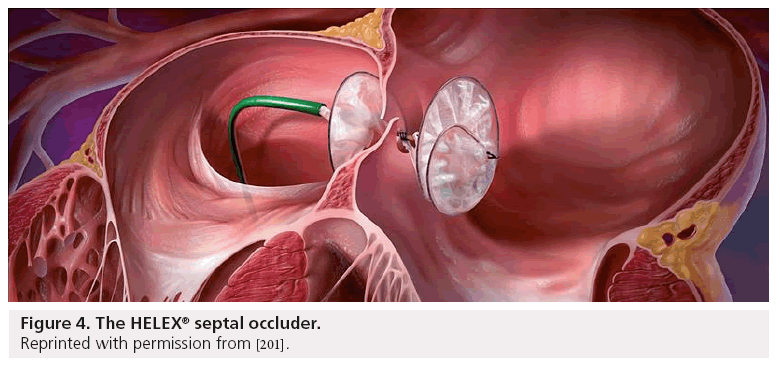
Atrial septal occluder. The AMPLATZER Septal Occluder is a transcatheter closure device used to treat ASDs. Device erosion has been associated with the AMPLATZER septal occluder in patients with retro-aortic rim deficiency. Atrial Septal Occluder Transcatheter Closure of Atrial Septal Defects and Patent Foramen Ovale.
Atrial septal occlusion devices are implantable cardiac devices used in patients with certain types of atrial septal defects. 423 patients received 433 devices with a total device exposure of 9115 years. The GORE CARDIOFORM Septal Occluder consists of an implantable Occluder and a Delivery System.
It is folded into a special delivery catheter similar to the catheter used to cross the heart defect during catheterization. The design of the Flex II ASD Occluder results in a septum alignment which increases its feasibility and patient safety during implantation. Between 2010 and 2014 125 patients underwent atrial septal defect closure with the CeraFlexTM septal occluder n 58 and the Amplatzerseptal occluder n 67 under transesophageal echocardiography guidance.
The reported rate is 051 which is. A variety of transcatheter atrial septal defect ASD occluders are currently in use around the world. Previously-approved ASD Closure devices have known limitations.
The AMPLATZER Septal Occluder was evaluated in a multi-center nonrandomized pivotal study comparing the device to surgical closure of atrial septal defects. Indiv idual patient exposure to the device averaged 256 months ranging from 0 to 389. The GORE Septal Occluder is a permanent implant delivered via a transcatheter approach.
The OFF-II is a. The Helex septal occluder is a new type of device designed to improve the results of transcatheter ASD closure. The Occluder is comprised of a platinum-filled nickel- titanium Nitinol wire frame covered with.
1 Recent studies found that transcatheter closure of isolated secundum atrial septal defects using the novel design of the Occlutech ASD Occluder was safe effective and had a closure rate of 973 with an excellent outcome during the 12-month follow-up period. They are used in cases of atrial septal defects with right atrial or ventricle enlargement to prevent paradoxical embolism left-to-right shunting and platypnea-orthodeoxia syndrome 1. Atrial septal defect ASD is among the most common congenital heart defects that require intervention and although the septal occluder device was approved in 2001 in the US.
GORE CARDIOFORM Septal Occluder - Product Overview Permanent transcatheter closure of atrial septal defects ASD and patent foramen ovale PFO Designed to perform with the natural anatomy of the heart the GORE CARDIOFORM Septal Occluder is a soft and conformable device for transcatheter closure of ASDs and PFOs up to 17 mm including challenging defects. Although for the most part effective all of these devices lack features that would be desirable in a perfect device. The stent is made of medical nickel-titanium memory alloy wires and filled with the polyester fiber membrane to be applied for the atrial septal defect.
Vascular Closure Devices and Thrombosis. MemoCarna Atrial Septal Defect ASD Occluder with single hub The product is composed of a nickel titanium alloy stent a stainless steel bushing and a polyester fiber membrane. It consists of two Nitinol wire mesh discs filled with polyester fabric.
Patient characteristics the stretched size of the defect device size and fluoroscopy time were similar between the groups. Cardiac Catheterization in Adult. Owning to the development of interventional therapy five types of occluders are now available on the market to deal with congenital or structural heart diseases atrial septal defect ASD occluders patent foramen ovale PFO occluders ventricular septal defect VSD occluders patent ductus arteriosus PDA occluders and left atrial appendage LAA occluders 6 7 8 9 10.
A permanently implanted device indicated for the percutaneous transcatheter closure of ostium secundum atrial septal defects ASDs The GORE CARDIOFORM ASD Occluders anatomically adaptable waist fills and conforms to each unique defect to close a broader range of ASDs. It is used to treat congenital heart defects of the atrial septum.
 Single Centre Experience With Gore Helex Septal Occluder For Closure Of Pfo Heart Lung And Circulation
Single Centre Experience With Gore Helex Septal Occluder For Closure Of Pfo Heart Lung And Circulation
 In Patients With Smaller Margins An Atrial Septal Occluder Device Download Scientific Diagram
In Patients With Smaller Margins An Atrial Septal Occluder Device Download Scientific Diagram
Transcatheter Closure Of Atrial Septal Defects Cardiac Interventions Today
Early Progressive Atrioventricular Block After Amplatzer Septal Occluder Closure Of A Large Atrial Septal Defect Scitemed Publishing Group
 Transcatheter Closure Of Atrial Septal Defect Principles And Available Devices Jung Journal Of Thoracic Disease
Transcatheter Closure Of Atrial Septal Defect Principles And Available Devices Jung Journal Of Thoracic Disease
 Gore Cardioform Asd Occluder Approved By Fda To Treat Atrial Septal Defects Medgadget
Gore Cardioform Asd Occluder Approved By Fda To Treat Atrial Septal Defects Medgadget
 Septal Occluder Cleared For Use In Very Young Patients Daic
Septal Occluder Cleared For Use In Very Young Patients Daic
 Fda Reports Serious Erosion Events With Amplatzer Septal Occluder Daic
Fda Reports Serious Erosion Events With Amplatzer Septal Occluder Daic
 Patent Foramen Ovale Atrial Septal Defect Ventricular Septal Defect Closure The Cardiology Advisor
Patent Foramen Ovale Atrial Septal Defect Ventricular Septal Defect Closure The Cardiology Advisor
 Selecting The Optimal Closure Device In Patients With Atrial Septal Defects And Patent Foramen Ovale
Selecting The Optimal Closure Device In Patients With Atrial Septal Defects And Patent Foramen Ovale
 Amplatzer Septal Occluder Aso Lateral Aspect Left And Right Discs Download Scientific Diagram
Amplatzer Septal Occluder Aso Lateral Aspect Left And Right Discs Download Scientific Diagram
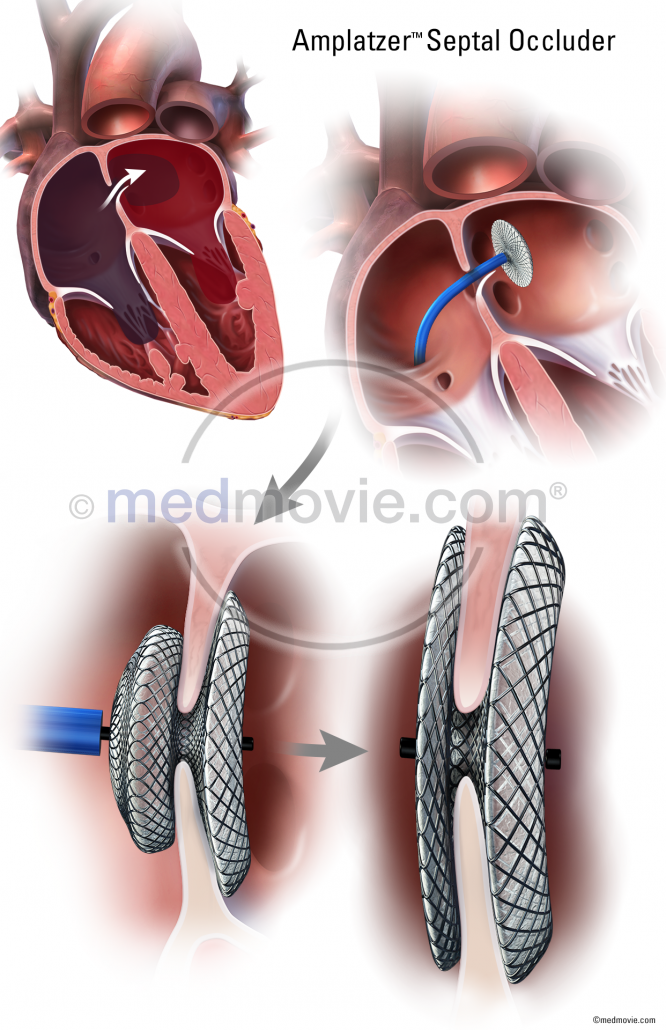 Amplatzer Septal Occluder Medmovie Com
Amplatzer Septal Occluder Medmovie Com
Atrial Septal Defect Asd Symptoms Causes Tests And Treatments
 Gore Cardioform Asd Occluder Receives Fda Approval For The Treatment Of Atrial Septal Defects Cath Lab Digest
Gore Cardioform Asd Occluder Receives Fda Approval For The Treatment Of Atrial Septal Defects Cath Lab Digest
Comments
Post a Comment