Featured
- Get link
- X
- Other Apps
Remicade Prescribing Information
REMICADE is indicated for reducing signs and symptoms of active arthritis inhibiting the progression of structural damage and improving physical function in adult patients with psoriatic arthritis PsA. REMICADE is indicated for reducing signs and symptoms in adult patients with active ankylosing spondylitis AS.
 Remicade Infliximab Injection Powder Lyophilized For Solution Prescription Rx Marketed Drugs Encyclopedia
Remicade Infliximab Injection Powder Lyophilized For Solution Prescription Rx Marketed Drugs Encyclopedia
Remicade is an antiinflammatory medicine.

Remicade prescribing information. Remicade is used with methotrexate a. The more common side effects of REMICADE include respiratory infections such as sinus infections and sore throat headache coughing and stomach pain. PREPARING FOR YOUR REMICADE INFUSION Your doctors office or infusion center will schedule your appointments.
REMICADE can lower the ability of your immune system to fight infections. Exercise caution when prescribing REMICADE for patients identified as carriers of HBV and monitor closely for active HBV infection during and following termination of therapy with REMICADE. Remicade is indicated for treatment of severely active ulcerative colitis in children and adolescents aged 6 to 17years who have had an inadequate response to conventional therapy including corticosteroidsand 6-MP or AZA or who are intolerant to or have medical contraindications for such.
Risk of infection REMICADE is a medicine that affects your immune system. Dated and received December 20 2018. Please read the full Prescribing Information and Medication Guide for REMICADE and discuss any questions you have with your doctor.
For detailed information about the REMICADE infusion process and questions please talk to. 5 mgkg at 0 2 and 6 weeks then every 8 weeks. Plaque Psoriasis REMICADE is indicated for treatment of adult patients with chronic severe ie extensive andor disabling.
What is the most important information I should know about REMICADE. These Prior Approval supplemental biologics applications provide for revisions to the US. Serious infections have happened in patients receiving REMICADE.
REMICADE is indicated for reducing signs and symptoms of active arthritis inhibiting the progression of structural damage and improving physical function in adult patients with psoriatic arthritis PsA. Patients with tuberculosis have frequently presented with disseminated or extrapulmonary disease. CLINICAL INFORMATION REMICADE is indicated for reducing signs and symptoms inhibiting the progression of structural damage and improving physical function in adult patients with moderately to severely active rheumatoid arthritis RA in combination with methotrexate MTX 1.
It is usually used when other medicines or treatments have failed in adults with the following diseases. The more common side effects of REMICADE include respiratory infections such as sinus infections and sore throat headache coughing and stomach pain. Please read the full Prescribing Information and Medication Guide for REMICADE and discuss any questions you have with your doctor.
Remicade infliximab for injection. Active tuberculosis including reactivation of latent tuberculosis. Exercise caution when considering resumption of REMICADE.
Discontinue REMICADE in patients who develop HBV reactivation and initiate antiviral therapy with appropriate supportive treatment. Food and Drug Administration. REMICADE is indicated for reducing signs and symptoms of active arthritis inhibiting the progression of structural damage and improving physical function in adult patients with psoriatic arthritis PsA.
Some adult patients who initially respond to treatment may benefit from increasing the dose to 10 REMICADE. If you need to reschedule an appointment call your doctors office or infusion provider. REMICADE infliximab Rheumatoid Arthritis.
REMICADE is indicated for reducing signs and symptoms of active arthritis inhibiting the progression of structural damage and improving physical function in adult patients with psoriatic arthritis PsA. Please view the full Prescribing Information including BOXED WARNING for REMICADE infliximab in English. REMICADE may cause serious side effects including.
See full prescribing information for Pediatric Ulcerative Colitis. For patients who test positive consult a physician with expertise in the treatment of hepatitis B. These infections include tuberculosis TB and.
Rheumatoid arthritis an immune system disease causing inflammation of the joints. Dated and received August 29 2018. Patients should be tested for latent tuberculosis before REMICADE use.
REMICADE is indicated for reducing signs and symptoms in adult patients with active ankylosing spondylitis AS. Dated and received March 5 2018 S-5391. REMICADE is indicated for reducing signs and symptoms in adult patients with active ankylosing spondylitis AS.
HIGHLIGHTS OF PRESCRIBING INFORMATION 5 mgkg at 0 2 and 6 weeks then every 8 weeks. REMICADE should be discontinued if a patient develops a serious infection or sepsis.
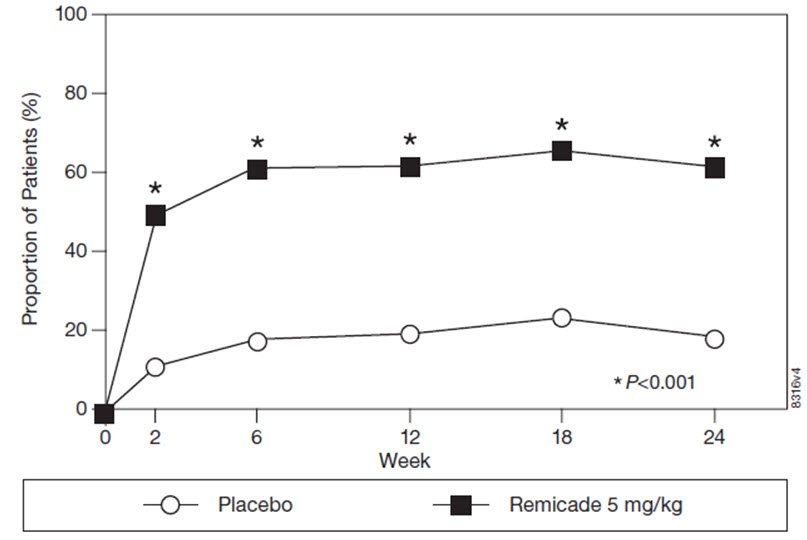 Remicade Fda Prescribing Information Side Effects And Uses
Remicade Fda Prescribing Information Side Effects And Uses
 Remicade Infliximab Fda Package Insert Drug Facts Iodine Com
Remicade Infliximab Fda Package Insert Drug Facts Iodine Com
 Additional Remicade Infliximab Resources Remicade
Additional Remicade Infliximab Resources Remicade
Official Consumer Website For Remicade Infliximab Remicade
 Additional Remicade Infliximab Resources Remicade
Additional Remicade Infliximab Resources Remicade

Https Www Janssenlabels Com Package Insert Product Monograph Prescribing Information Remicade Pi Pdf
Https Www Ema Europa Eu Documents Public Statement Emea Public Statement Infliximab Remicade Update Safety Concerns En Pdf

Https Www Wellrx Com Remicade Monographs
 These Highlights Do Not Include All The Information Needed To Use Remicade Safely And Effectively See Full Prescribing Information For Remicade Remicade Infliximab For Injection For Intravenous Use Initial U S Approval 1998
These Highlights Do Not Include All The Information Needed To Use Remicade Safely And Effectively See Full Prescribing Information For Remicade Remicade Infliximab For Injection For Intravenous Use Initial U S Approval 1998
Https Www Evicore Com Media Files Evicore Clinical Guidelines Solution Specialty Drugs Infliximab Remicade Inflectra Renflexis Eff100120 Pdf
Remicade Infliximab Hcp Information For Healthcare Professionals
Comments
Post a Comment