Featured
What Is Eua Stand For
Instead the FDA-issued EUA Fact Sheet for Recipients and Caregivers for each COVID-19 vaccine must be used. The term Emergency Use Authorization EUAis being used a lot.
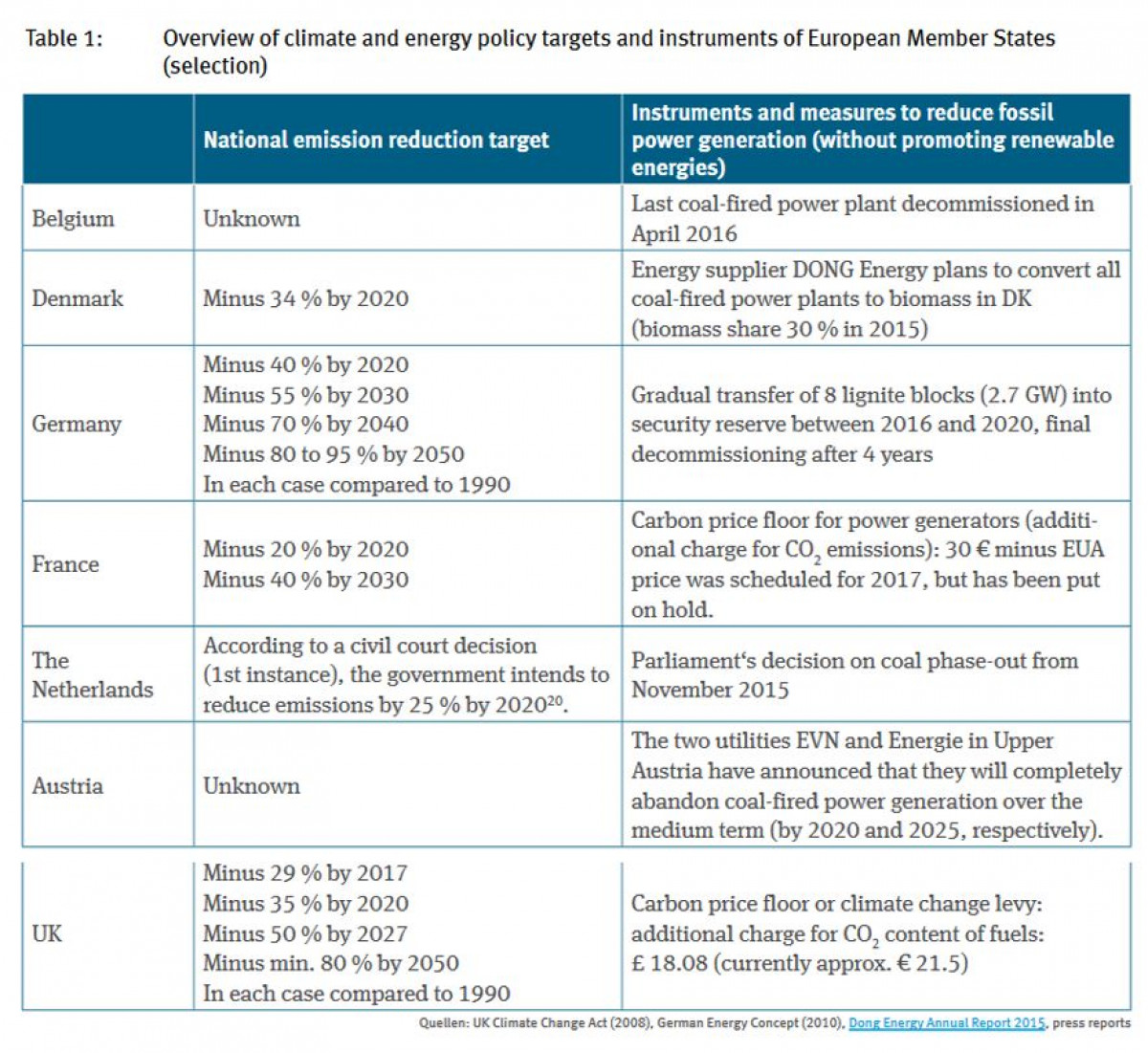 National Climate Measures And European Emission Trading Assessing The Waterbed Effect Clean Energy Wire
National Climate Measures And European Emission Trading Assessing The Waterbed Effect Clean Energy Wire
We know 83 definitions for EUA abbreviation or acronym in 6 categories.

What is eua stand for. One such opportunity is to partner with the US FDA by submitting an Emergency Use Authorization EUA for any products that could immediately and effectively address a health emergency. An Emergency Use Authorization EUA is a mechanism to facilitate the availability and use of medical countermeasures including vaccines during public. What does EUA mean.
Estats Units dAmèrica Català EUA. Add to My List Edit this Entry Rate it. Estados Unidos da América.
A public health threat exists. An Emergency Use Authorization EUA allows the US. Click here for a free PDF of our recommended tips for receiving EUA from FDA.
Please look for them carefully. Food and Drug Administration FDA to make a product or drug whether new or not yet proven for a given use available during an emergency provided there are data to suggest that it is reasonably safe and effective. For EUA we have found 83 definitions.
All acronyms 83 Airports Locations 3 Business Finance 3 Common. The basic requirements for an EUA are. Emergency Use Authorization US CDC EUA.
Electric Utilities Act Canada EUA. To help facilitate documentation of having provided the EUA Fact Sheet in electronic medical recordsimmunization information systems CDC is leveraging the existing VIS Code. In this article well go over the basics of what the EUA program is and how medical device companies can receive EUA for a product to be used in the fight against COVID-19.
As a result the FDA is authorized to grant Emergency Use Authorizations EUAs for any medical countermeasure MCM that facilitates the diagnosis prevention or. Heres what it means in relation to COVID-19. There is no VIS for COVID-19 vaccines authorized under an EUA.
The COVID-19 vaccines from Pfizer Moderna and Johnson Johnson have all received an Emergency Use Authorization EUA from the FDA. EUA Any operative or invasive procedure done while the patient is sedated in order to improve patient tolerance alleviate pain or anxiety or improve the quality of the exam. European Union Allowance CO2 emissions EUA.
What does EUA mean in FDA. 500 2 votes. Estados Unidos da América.
Possible EUA meaning as an acronym abbreviation shorthand or slang term vary from category to category. An EUA is a special designation that allows the US Food and Drug Administration FDA to help strengthen the countrys public health protections against chemical biological radiological and. EUAs may be granted only during a public health emergency and when there are no adequate approved and available alternatives.
This page is about the meanings of the acronymabbreviationshorthand EUA in the Governmental field in general and in the FDA terminology in particular. European Union Allowance EUA means the tradable unit under the European Union Emissions Trading Scheme EU ETS giving the holder the right to emit one tonne of carbon dioxide CO2 or the equivalent amount of two more powerful greenhouse gases nitrous oxide N2O and perfluorocarbons PFCs.
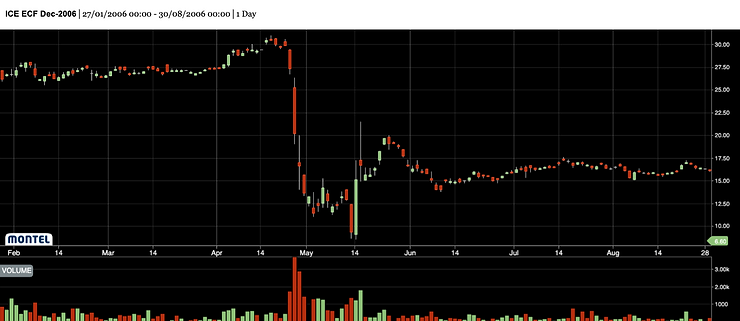 Comment The Mystery Of The 2006 Eua Price Crash Carbon Pulse
Comment The Mystery Of The 2006 Eua Price Crash Carbon Pulse
 Emergency Use Authorization What Does That Mean Integracare
Emergency Use Authorization What Does That Mean Integracare
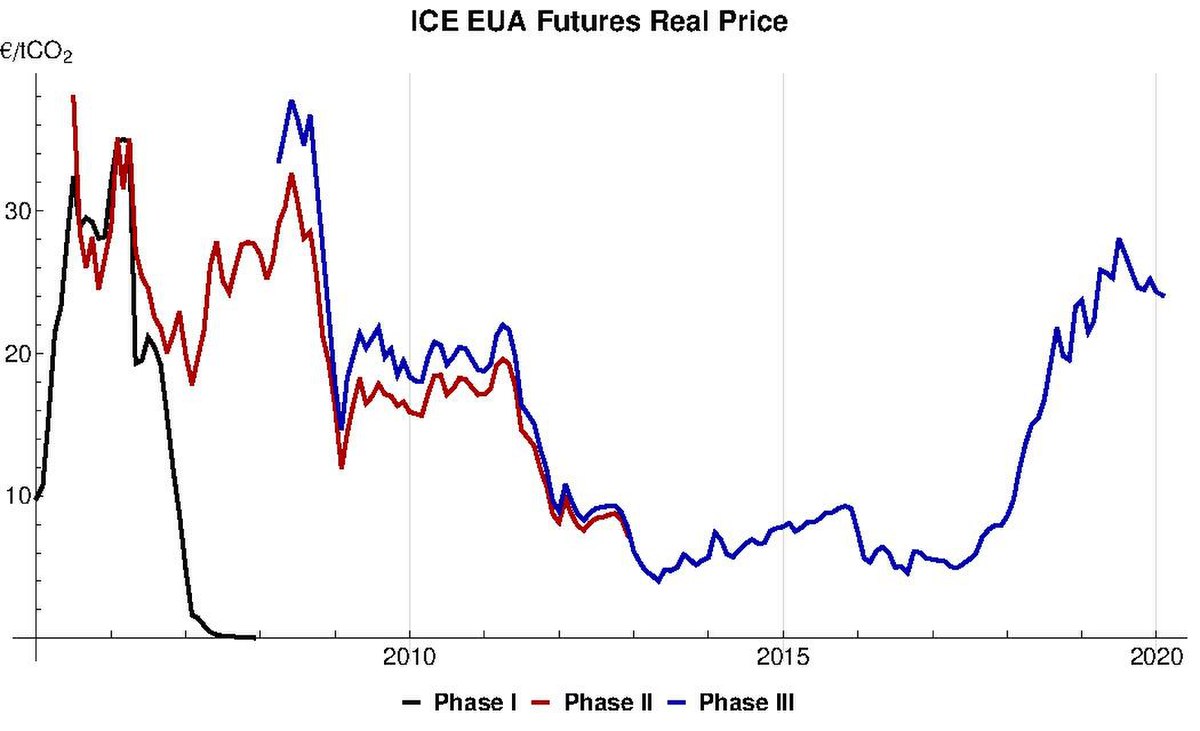 European Union Emission Trading Scheme Wikipedia
European Union Emission Trading Scheme Wikipedia
 What Is An Eua Pfizer Moderna Seek Emergency Use Authorization For Covid 19 Vaccines Health Com
What Is An Eua Pfizer Moderna Seek Emergency Use Authorization For Covid 19 Vaccines Health Com
 What S The Difference Between Fda Emergency Use Authorization And Fda Approval Unc Health Covid 19 Vaccine Hub
What S The Difference Between Fda Emergency Use Authorization And Fda Approval Unc Health Covid 19 Vaccine Hub
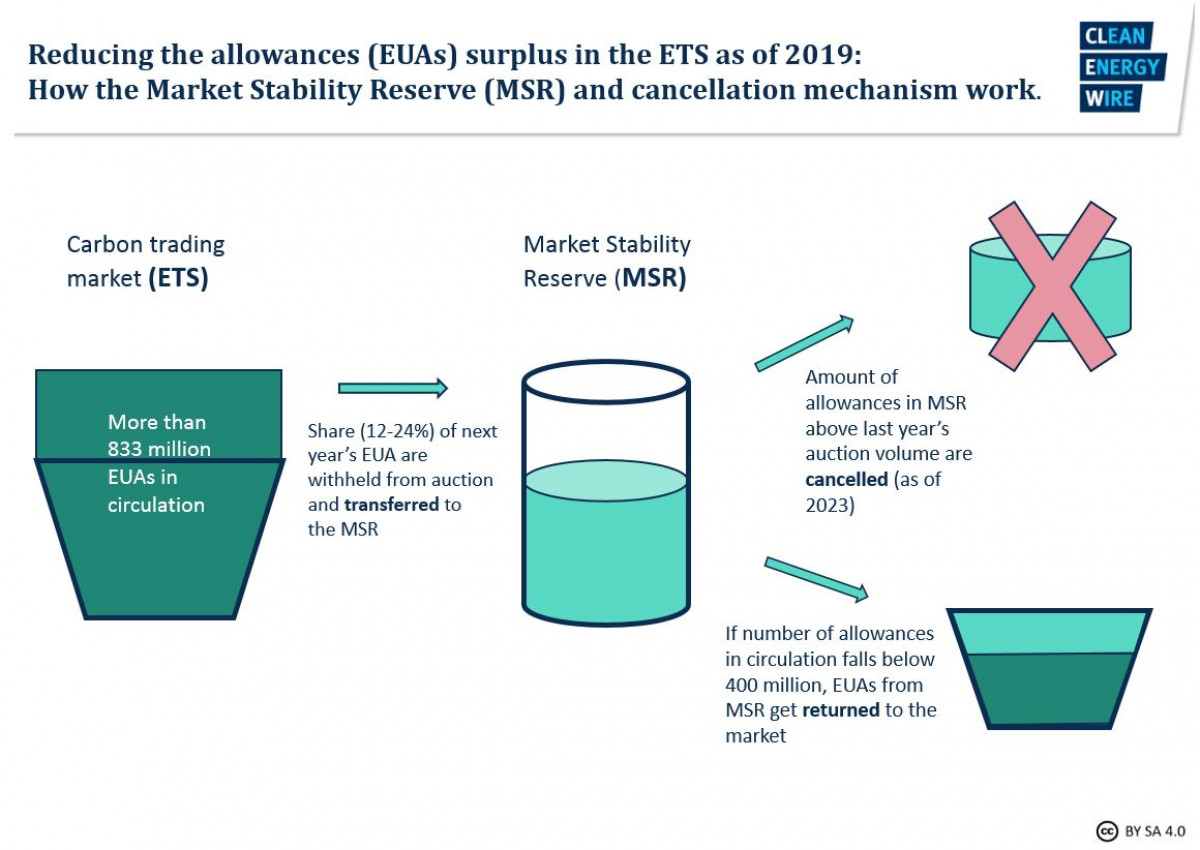 National Climate Measures And European Emission Trading Assessing The Waterbed Effect Clean Energy Wire
National Climate Measures And European Emission Trading Assessing The Waterbed Effect Clean Energy Wire
What Is Emergency Use Authorization Eua
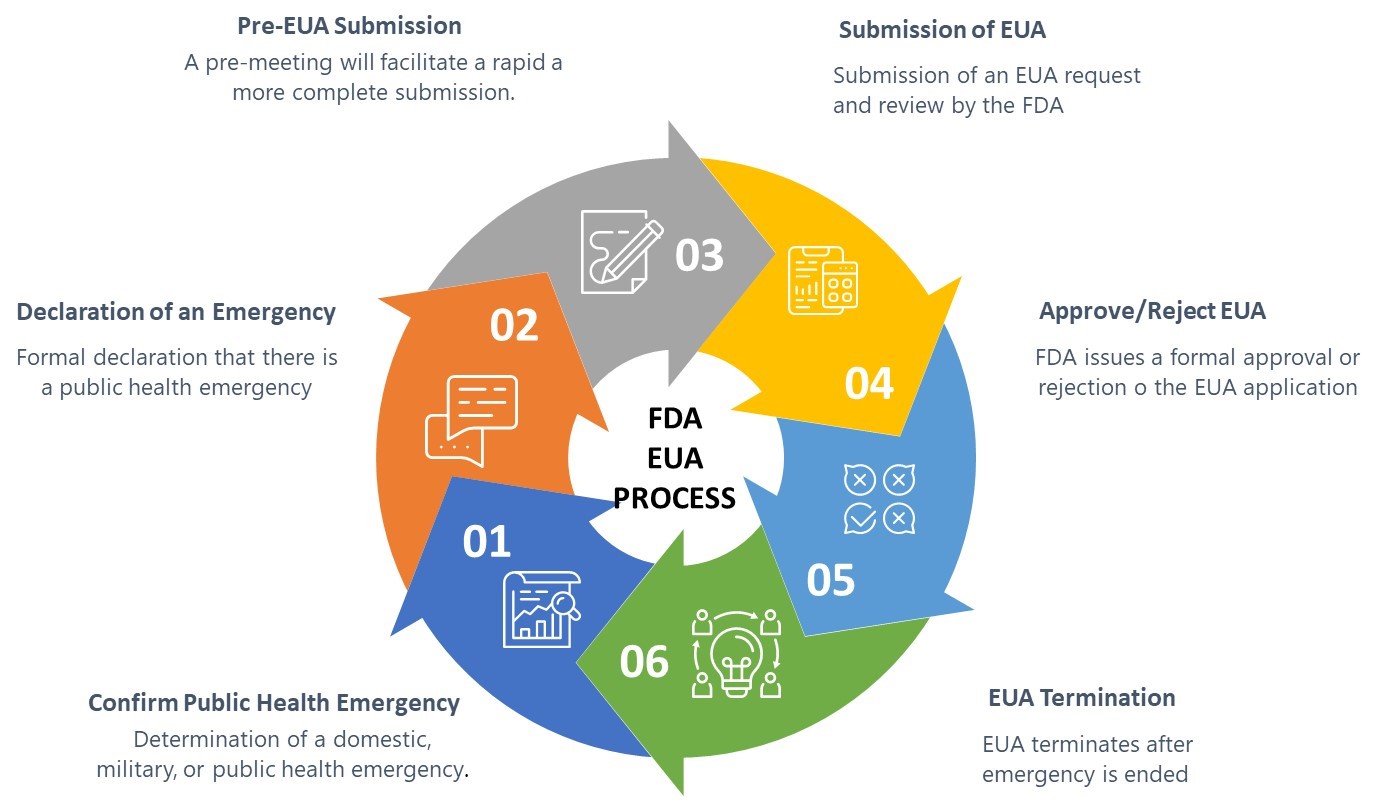 Navigating The Fda S Emergency Use Authorization Process
Navigating The Fda S Emergency Use Authorization Process
 What Are Euas For Coronavirus Vaccines And Treatments
What Are Euas For Coronavirus Vaccines And Treatments
 Eua 101 What Is Emergency Use Authorization And How Can My Device Get Authorized
Eua 101 What Is Emergency Use Authorization And How Can My Device Get Authorized
Https Eua Eu Component Attachments Attachments Html Id 414
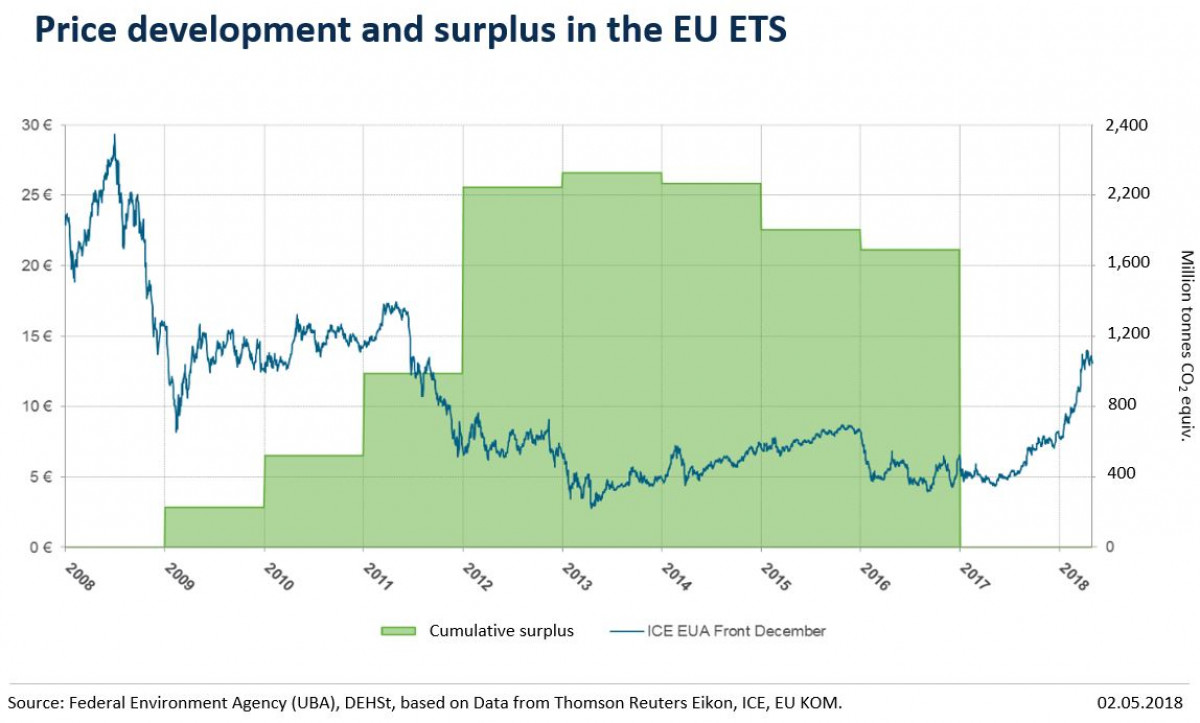 National Climate Measures And European Emission Trading Assessing The Waterbed Effect Clean Energy Wire
National Climate Measures And European Emission Trading Assessing The Waterbed Effect Clean Energy Wire

Comments
Post a Comment