Featured
Stelara Trough Levels
In the derivation analysis baseline albumin gL HR 1041. In those with an endoscopic response the mean trough concentration of ustekinumab was 47 mcgmL compared with 38 mcgmL those without an endoscopic response.
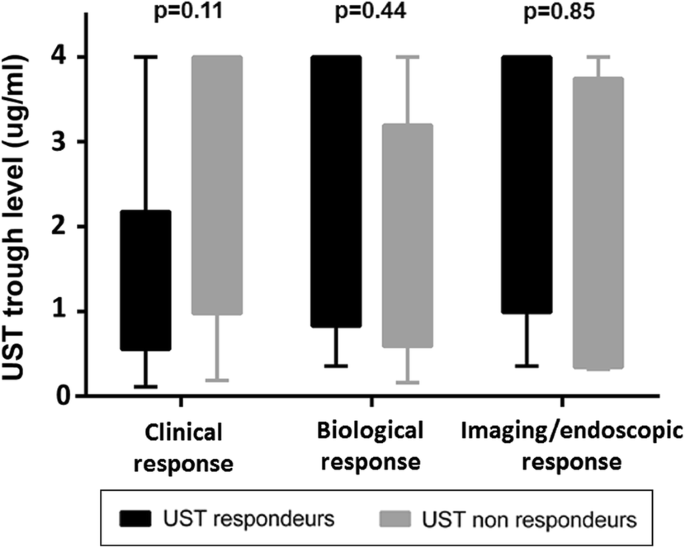 Ustekinumab Serum Trough Levels May Identify Suboptimal Responders To Ustekinumab In Crohn S Disease Springerlink
Ustekinumab Serum Trough Levels May Identify Suboptimal Responders To Ustekinumab In Crohn S Disease Springerlink
1 - 6 months.

Stelara trough levels. Among patients who had an HBI 4 a level of CRP 5mgdL a level of fecal calprotectin 250ugg or endoscopic evidence for disease activity before dose interval shortening after the dose interval was shortened 28 achieved clinical remission an HBI score 4 22 had a normal level of CRP levels of fecal calprotectin and 36 achieved endoscopic remission. The median trough concentrations in the STELARA 90 mg q8w group were approximately threefold greater than in the 90 mg q12w group. STELARA Inf Konz 130 mg26ml.
Cautions Discusses conditions that may cause diagnostic confusion including improper specimen collection and handling inappropriate test. . Announces a new therapeutic drug monitoring test for use in adult inflammatory bowel disease IBD patients treated with Stelara ustekinumab.
PM-2015-04746-1-1 Final 24 October 2017. 36 Given that most samples were not trough levels. An 8-week trough level of 20 mcgmL was found to be optimal and distinguished primary nonresponders from responders with a sensitivity of 87 a specificity of 66 a positive predictive value of 82 and a negative predictive value of 75.
Specifically the median preadministration ustekinumab concentrations were consistent through week 44 for both 90 mg q8w group range. Concentrations or 10 mcgmL are. Stelara is administered by injection under the skin subcutaneous.
Week 28 increased with increasing serum ustekinumab trough levels at Week 28. This test 1 measures infliximab and infliximab-dyyb inflectra levels to help. Time on Stelara when people have Blood test abnormal.
20 mgmL to 22 mgmL at IM-UNITI weeks 8 16 24 32 and 40 and 90 mg q12w group range. Limit of quantitation is 03 mcgmL. Minimum trough immediately before next infusion therapeutic concentrations of vedolizumab are expected to be above 15 mcgmL.
6 - 12 months. SAN DIEGO CA October 16 2017 Prometheus Laboratories Inc. This Product Information was approved at the time this.
2 - 5 years. New Test Monitors Therapeutic Ustekinumab Levels in IBD Patients. Antibody to STELARA rates through week 272 remained low.
A greater proportion of patients with trough concentrations of ustekinumab above 45 μgmL at week 26 or later had an endoscopic response 759 than did patients with trough concentrations below this level 407. PROMETHEUS Anser UST enables physicians to monitor. We used univariate logistic regression models with the 6-month response data set to explore the association between early drug levels and treatment response in the presence of other covariates including those previously identified as factors associated with response in the BADBIR cohort eg weight raceethnicity disease and treatment duration ustekinumab dose and biologic-naïve status.
Infliximab Level and Anti-drug Antibody for IBD - When treatment of inflammatory bowel disease with infliximab or its biosimilar fails a physician may need to consider treatment options such as adjusting dose or dosing intervals switching to a different TNF blocker or switching to a non-TNF blocker. The dosage and frequency of administration depend on the condition being treated. In psoriatic arthritis studies.
1 - 2 years. Product information for AusPAR Stelara Ustekinumab Janssen-Cilag Pty. Random drug levels may be ordered and are appropriate for monitoring drug usage if drug is.
95 CI 09951527 absence of baseline actively draining fistula. After appropriate training people can be taught how to self-administer Stelara or caregivers shown how to give it to another person. 3 Zeilen STELARA 90 mg SC q8w.
The peak level is the highest concentration of a drug in the patients bloodstream. 06 mgmL to 08. Using a trough threshold of 45 mcgmL at week 26 759 had an endoscopic response whereas those with a level below this trough had a 407 endoscopic response.
Among 23 patients with lab data available median ustekinumab troughs were 13 μg before escalation began and other indicators of inflammation were elevated as well including a median. 95 CI 10191063 no prior smoking history HR 1233. Steering clear of the days major pivot levels however would support further upside ahead.
What initial blood tests are required before Stelara is given. The trough level is the lowest concentration in the patients bloodstream therefore the specimen should be collected just prior to administration of the drug. 5 - 10 years.
Patients with trough concentrations of ustekinumab above 45 μgmL at week 26 or later also had a lower mean level of CRP 126 mgL than did patients with trough concentrations. In inflammatory bowel disease at post-induction measurement week 8 concentrations above35 mcgmL are associated with good outcomes. Normalized CRP at week 92 nN.
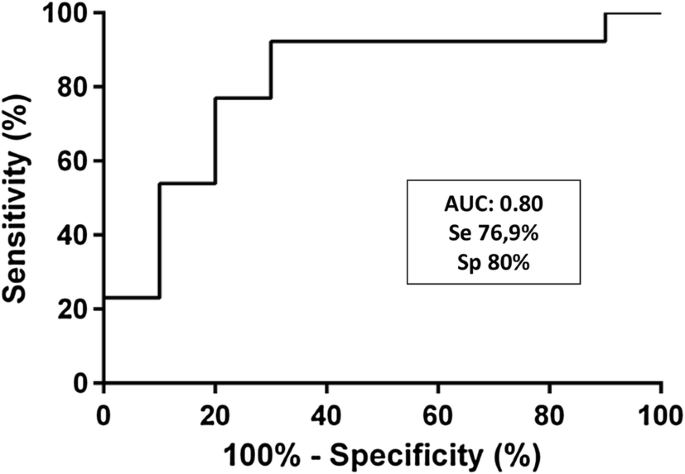 Ustekinumab Serum Trough Levels May Identify Suboptimal Responders To Ustekinumab In Crohn S Disease Springerlink
Ustekinumab Serum Trough Levels May Identify Suboptimal Responders To Ustekinumab In Crohn S Disease Springerlink
Https Www Ema Europa Eu En Documents Variation Report Stelara H C 958 Ii 0073 Epar Assessment Report Variation En Pdf
 Median Serum Ustekinumab Concentrations Over Time From Induction With A Download Scientific Diagram
Median Serum Ustekinumab Concentrations Over Time From Induction With A Download Scientific Diagram
Https Www Ema Europa Eu Documents Variation Report Stelara H C 958 Ii 0029 Epar Assessment Report Variation En Pdf
Https Www Ema Europa Eu En Documents Variation Report Stelara H C 958 Ii 0073 Epar Assessment Report Variation En Pdf
Https Www Ema Europa Eu Documents Variation Report Stelara H C 958 Ii 0029 Epar Assessment Report Variation En Pdf
Https Www Gastrojournal Org Article S0016 5085 18 30111 2 Pdf
Https Www Ema Europa Eu En Documents Variation Report Stelara H C 958 Ii 0042 Epar Assessment Report Variation En Pdf
 Therapeutic Drug Monitoring Ustekinumab Stelara Gutsandgrowth
Therapeutic Drug Monitoring Ustekinumab Stelara Gutsandgrowth
 Ustekinumab Pharmacokinetics And Exposure Response In A Phase 3 Randomized Trial Of Patients With Ulcerative Colitis Sciencedirect
Ustekinumab Pharmacokinetics And Exposure Response In A Phase 3 Randomized Trial Of Patients With Ulcerative Colitis Sciencedirect
Https Www Ema Europa Eu Documents Variation Report Stelara H C 000958 X 0049 G Epar Assessment Report Extension En Pdf
 Association Between Ustekinumab Trough Concentrations And Clinical Biomarker And Endoscopic Outcomes In Patients With Crohn S Disease Sciencedirect
Association Between Ustekinumab Trough Concentrations And Clinical Biomarker And Endoscopic Outcomes In Patients With Crohn S Disease Sciencedirect
Https Www Gastrojournal Org Article S0016 5085 18 30111 2 Pdf
Https Www Gastrojournal Org Article S0016 5085 18 30111 2 Pdf
Comments
Post a Comment