Featured
Guardant Liquid Biopsy
The Guardant360 assay is a breakthrough liquid biopsy that provides fast accurate and comprehensive genomic results from a simple blood draw in approximately seven days upon receipt in the US laboratory. 1 The test is broadly covered by.
 Guardant Health Launches Guardant Reveal Liquid Biopsy Test For Residual Disease And Recurrence Monitoring In Patients With Early Stage Colorectal Cancer Business Wire
Guardant Health Launches Guardant Reveal Liquid Biopsy Test For Residual Disease And Recurrence Monitoring In Patients With Early Stage Colorectal Cancer Business Wire
These tests fuel development of its LUNAR screening program which aims to address the needs of asymptomatic individuals eligible for cancer screening and.
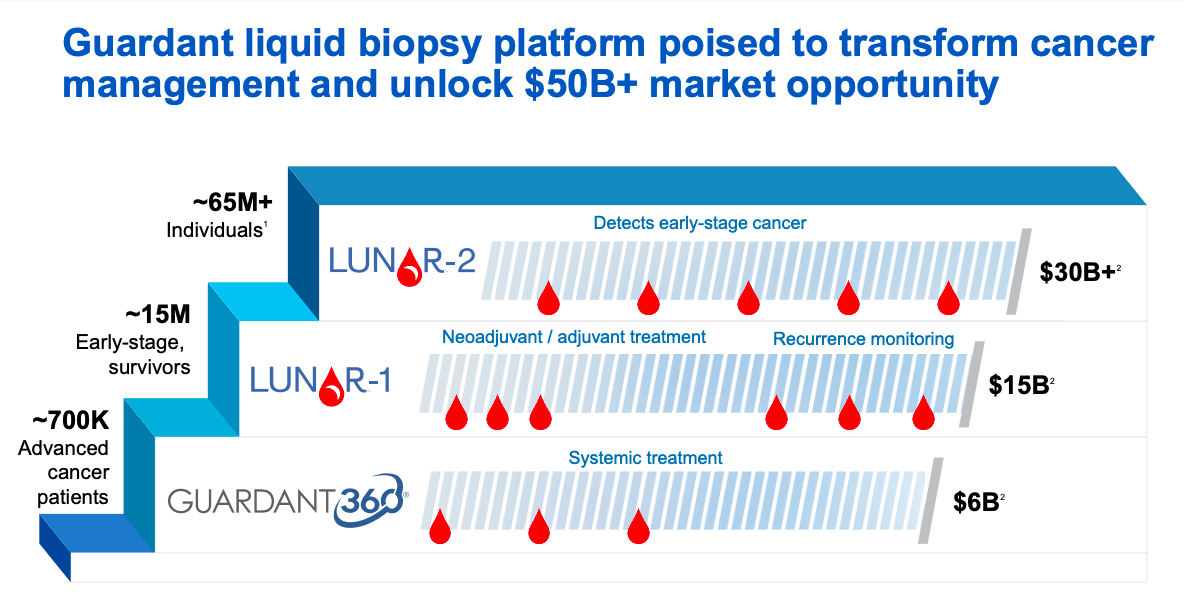
Guardant liquid biopsy. Conquering Cancer with Data. These tests fuel development of its LUNAR screening program which aims to address the needs of asymptomatic individuals. Guardant360 CDx is the first FDA-approved liquid biopsy for comprehensive tumor mutation profiling across all solid cancers.
Guardant Health Inc. A new study in JCO Precision Oncology shows the Guardant360 liquid biopsy test provides an early indication of treatment response to pembrolizumab-based immunotherapy by detecting molecular. Guardant Health has commercially launched liquid biopsy-based Guardant360 Guardant360 CDx and GuardantOMNI tests for advanced stage cancer patients and Guardant Reveal test for early-stage cancer patients.
Since its launch in 2014 the Guardant360 assay has been ordered by more than 7000 oncologists over 150000 times for patients with advanced cancer to help. The Guardant360 assay is Guardant Healths breakthrough liquid biopsy that provides fast accurate and comprehensive genomic results from a simple blood draw in approximately seven days upon sample receipt in the laboratory. The first is called liquid biopsy which uses a blood sample to provide health care professionals with genetic information about the patients.
1 day agoGuardant Health has commercially launched liquid biopsy-based Guardant360 Guardant360 CDx and GuardantOMNI tests for advanced stage cancer patients and Guardant Reveal test for early-stage cancer patients. Guardant Health has commercially launched liquid biopsy-based Guardant360 Guardant360 CDx and GuardantOMNI tests for advanced stage cancer patients and Guardant Reveal for early-stage cancer patients. 4 rows The Food and Drug Administration FDA has approved two blood tests known as liquid biopsies.
COVID-19 Update How Guardant Health is supporting cancer care during the pandemic. Guardant Health has commercially launched liquid biopsy-based Guardant360 Guardant360 CDx and GuardantOMNI tests for advanced stage cancer patients and Guardant Reveal for early-stage cancer patients. Guardant Health has commercially launched liquid biopsy-based Guardant360 Guardant360 CDx and GuardantOMNI tests for advanced stage cancer patients and Guardant Reveal test for early.
1 day agoGuardant Health Presents Data at 2021 ASCO Annual Meeting Showing Utility of Liquid Biopsy in Early- and Late-Stage Cancers. Guardant Health has commercially launched liquid biopsy-based Guardant360 Guardant360 CDx and GuardantOMNI tests for advanced stage cancer patients and Guardant Reveal test for early-stage cancer patients. The Guardant360 CDx assay utilizes two technologies.
Data from 20 abstracts demonstrate value of companys blood tests. 1 It has been used by more than 7000 oncologists nationwide and more than 150000 Guardant360 tests have been performed to date. Guardant360 Liquid Biopsy Assay Guardant Health.
GH to present data demonstrating the use of the companys proprietary blood tests to advance precision oncology including. These tests fuel development of its LUNAR screening program which aims to address the needs of asymptomatic individuals eligible for. Since being introduced as a laboratory developed test LDT the Guardant360 liquid biopsy LDT has become widely accepted for blood-based CGP with more than 150 peer-reviewed publications.
These tests fuel development of its LUNAR screening program which aims to address the needs of asymptomatic individuals eligible for cancer screening and. The Guardant Health Oncology Platform leverages capabilities to drive commercial adoption improve patient clinical outcomes and lower healthcare costs across all stages of the cancer care. These tests fuel development of its LUNAR screening program which aims to address the needs of asymptomatic individuals.
It is used for advanced stage cancer patients with solid tumors.
 Guardant Pips Roche To Liquid Biopsy Approval Evaluate
Guardant Pips Roche To Liquid Biopsy Approval Evaluate
 Priority Health Becomes First Health Plan To Cover Guardant Health S Comprehensive Liquid Biopsy Exosome Rna
Priority Health Becomes First Health Plan To Cover Guardant Health S Comprehensive Liquid Biopsy Exosome Rna
Guardant Health To Present Liquid Biopsy Data From More Than 15 000 Patients At Asco Annual Meeting
 Priority Health Becomes First Health Plan To Cover Guardant Health S Comprehensive Liquid Biopsy Exosome Rna
Priority Health Becomes First Health Plan To Cover Guardant Health S Comprehensive Liquid Biopsy Exosome Rna
 Guardant Health Looks Forward To The Liquid Biopsy Opportunity In 2019 Nasdaq Gh Seeking Alpha
Guardant Health Looks Forward To The Liquid Biopsy Opportunity In 2019 Nasdaq Gh Seeking Alpha
 Guardant S Liquid Biopsy Matches Tissue Testing In Lung Cancer Evaluate
Guardant S Liquid Biopsy Matches Tissue Testing In Lung Cancer Evaluate
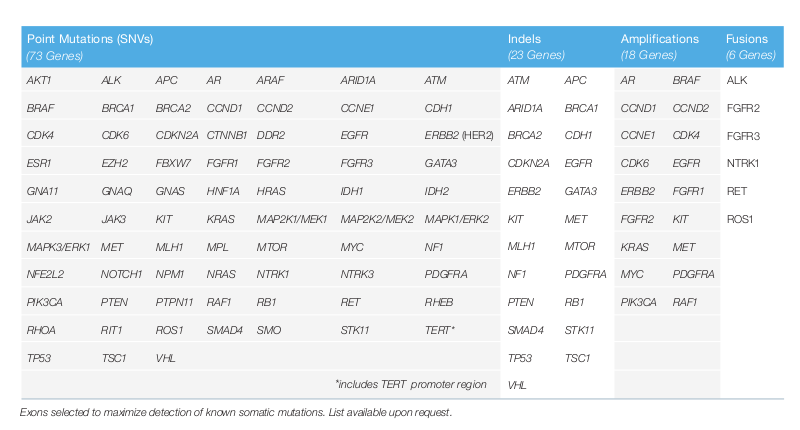 Guardant360 Therapy Planning With Blood Liquid Biopsy Therapyselect
Guardant360 Therapy Planning With Blood Liquid Biopsy Therapyselect
 Guardant Health Looks Forward To The Liquid Biopsy Opportunity In 2019 Nasdaq Gh Seeking Alpha
Guardant Health Looks Forward To The Liquid Biopsy Opportunity In 2019 Nasdaq Gh Seeking Alpha
 Guardant Health A Rising Star Nasdaq Gh Seeking Alpha
Guardant Health A Rising Star Nasdaq Gh Seeking Alpha
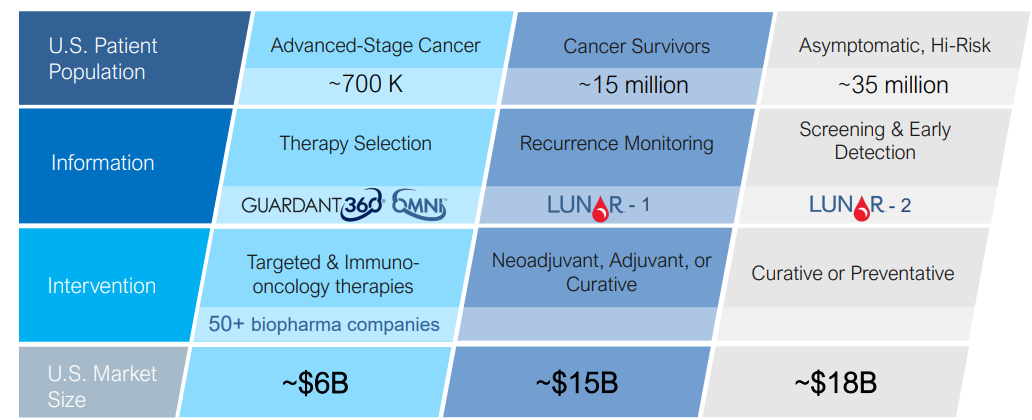 Guardant Health Bright Future For Liquid Biopsy Leader But Valuation Is Rich Nasdaq Gh Seeking Alpha
Guardant Health Bright Future For Liquid Biopsy Leader But Valuation Is Rich Nasdaq Gh Seeking Alpha
 Guardant360 Liquid Biopsy Quickly Identifies Targetable Mutations In Breast Cancer 2019 12 19 Bioworld
Guardant360 Liquid Biopsy Quickly Identifies Targetable Mutations In Breast Cancer 2019 12 19 Bioworld
 Guardant360 Therapy Planning With Blood Liquid Biopsy Therapyselect
Guardant360 Therapy Planning With Blood Liquid Biopsy Therapyselect
 Jpmvfinalhe1132020v2 Guardant Health Inc 2020 Current Report 8 K
Jpmvfinalhe1132020v2 Guardant Health Inc 2020 Current Report 8 K
 Guardant Guardant360 Liquid Biopsy Assay Guardant Health
Guardant Guardant360 Liquid Biopsy Assay Guardant Health
Comments
Post a Comment